Welcome to the e-CCO Library Archive!
DOP019
Efficacy of autologous haematopoietic stem cell transplantation for refractory Crohn’s disease
A. López García1, M. Rovira2, A. Jauregui Amezaga1, P. Marin3, A. Salas1, S. Pinó Donnay1, R. Barastegui1, F. Feu1, J. Elizalde1, F. Fernández-Avilés2, C. Martínez4, G. Gutierrez2, L. Rosiñol2, E. Carreras2, A. Urbano2, M. Lozano3, J. Cid3, M. Suárez-Lledó2, J. Mensa5, J. Rimola6, S. Rodriguez6, M. C. Masamunt1, D. Comas1, A. Ramirez Morros1, M. Gallego1, I. Ordás1, J. Panés1, E. Ricart*1
1Hospital Clinic Barcelona, Gastroenterology, Barcelona, Spain, 2Hospital Clinic Barcelona, Haematology, Barcelona, Spain, 3Hospital Clinic Barcelona, Biomedic Diagnostic Centre, Barcelona, Spain, 4Hospital Clinic Barcelona, Ginecology, Barcelona, Spain, 5Hospital Clinic Barcelona, Internal Medicine, Barcelona, Spain, 6Hospital Clinic Barcelona, Radiology, Barcelona, Spain
DOP020
Prediction of clinical and endoscopic remission after autologous stem cell transplantation in treatment refractory Crohn’s disease: pooled results from the ASTIC trial
J. Lindsay*1, M. Allez2, M. Clark3, M. Labopin4, E. Ricart5, G. Rogler6, M. Rovira7, J. Satsangi8, D. Farge9, C. Hawkey3
1Blizard Institute, Barts and the London School of Medicine, Centre for Immunobiology, London, United Kingdom, 2APHP St. Antoine Hospital, Department of Gastroenterology, Paris, France, 3Nottingham Digestive Diseases Centre, Department of Digestive Diseases, Biomedical Research Unit, Nottingham, United Kingdom, 4European Group for Blood and Marrow Transplantation (EBMT), Paris, France, 5Hospital Clinic Barcelona, Gastroenterology, Barcelona, Spain, 6University Hospital Zürich, Department of Gastroenterology and Hepatology, Zürich, Switzerland, 7Hospital Clinic de Barcelona, Department of Haematology, Barcelona, Spain, 8Western General Hospital, Gastrointestinal Unit, Edinburgh, United Kingdom, 9Hospital Saint-Louis, Department of Internal Medicine and Vascular Pathology, Inserm U 976, Paris, France
DOP021
Long-term efficacy of autologous haematopoietic stem cells transplantation for refractory Crohn’s disease: 10 years of Milan experience without CD34+ cell selection
A. Cassinotti*1, F. Onida2, C. Annaloro2, G. Saporiti2, M. Fichera1, M. Daperno3, B. Motta2, P. Fociani4, E. Tagliaferri2, G. Sampietro5, D. Vincenti2, A. Gregorini2, G. Maconi1, M. Nebuloni6, A. Cortelezzi2, S. Ardizzone1
1Gastroenterology Unit, Luigi Sacco University Hospital, Milan, Italy, 2Bone Marrow Transplantation Centre, Fondazione Ospedale Maggiore Policlinico, Mangiagalli e Regina Elena, University of Milan, Milan, Italy, 3Gastroenterology Unit, AO Ordine Mauriziano, Turin, Italy, 4Luigi Sacco University Hospital, Pathology Unit, Milan, Italy, 52nd Division of Surgery, Luigi Sacco University Hospital, Milan, Italy, 6Pathology Unit, Luigi Sacco University Hospital, Milan, Italy
DOP022
Targeting immune cell metabolism: LYC-30937, a novel therapeutic approach for inflammatory bowel disease
L. Carter1, R. Morgan1, C. Lesch1, M. Spahr1, L. Franchi2, I. Monteleone3, G. Monteleone3, G. Glick2, H. J. Wilkins1, P. Higgins*2
1Lycera, Ann Arbor, Michigan, United States, 2University of Michigan, Ann Arbor, Michigan, United States, 3Tor Vergata, Rome, Italy
DOP023
Safety and efficacy of a novel IV targeted pegylated liposomal prednisolone product (Nanocort): results from a phase 2a study in patients with active ulcerative colitis
G. van Assche1, P. Rutgeerts1, M. Ferrante1, M. Noman1, H. Fidder2, B. Oldenburg2, J. Metselaar3, 4, S. Vermeire*1
1University Hospitals Leuven, Department of Gastroenterology, Leuven, Belgium, 2University Medical Centre Utrecht, Department of Gastroenterology and Hepatology, Utrecht, Netherlands, 3Enceladus Pharmaceuticals, Naarden, Netherlands, 4University Clinic RWTH, Experimental Molecular Imaging, Aachen, Germany
Ulcerative Colitis (UC) is a chronic, relapsing inflammatory disease affecting the mucosal lining of the rectum and the colon to a variable extent. Corticosteroids have long been a cornerstone in the treatment of UC, despite considerable side effects including Cushingoid facies and weight gain, acne, hyperglycaemia, insomnia, infections, and osteoporosis after extended use. Nanocort is a novel pharmaceutical composed of prednisolone sodium phosphate enclosed in 100 nm PEGylated liposomes, which, after IV infusion, selectively target and accumulate in inflamed bowel lesions and selectively deliver high and effective concentrations of corticosteroids, thus reducing the required total steroid dose and dosing frequency.
In an exploratory, 2-centre (neoplastic lesions [NL] and BE), randomised, placebo-controlled, observer-blind phase 2a study, the safety and efficacy of Nanocort was evaluated in 18 patients aged 22–63 with moderate-to-severe active UC. Two IV doses of 150 mg Nanocort (n = 14) or saline (n = 4) were given as slow infusions 2 weeks apart. Overall safety (primary endpoint), pharmacokinetics, and efficacy were assessed at weeks 2, 4, 8, and 12 after start of treatment.
The median total Mayo score (tMS, 0–12) at baseline was 10 (range 7–12). At week 4 the Nanocort group showed clear benefit in 70% of the patients with 4 patients out of 13 in remission. Further, 7 out of 13 patients showed a reduction of the endoscopy sub score of ≥ 1 point reaching ≤ 1 point. Rapid effects on partial Mayo score (pMS, 0–9) were shown with persisting remissions in 7/13 patients treated with Nanocort. Remission was defined as a post-treatment MS of ≤ 2 points with all sub scores ≤ 1 point. Typical steroid-related adverse effects were sparse with isolated mild to moderate cases of acne, dyspepsia, nausea, and gastritis. No significant suppression of urinary cortisol was found, nor were there any indications of hyperglycaemia. Some patients experienced infusion reactions probably related to the trial medication. One patient experienced an exacerbation of psoriasis upon withdrawal.
The results of this phase 2a study demonstrate that IV Nanocort can be a safe new therapy with fast and durable therapeutic benefit for patients with active UC without the drawbacks of oral steroid standard-of-care. Larger studies are warranted.
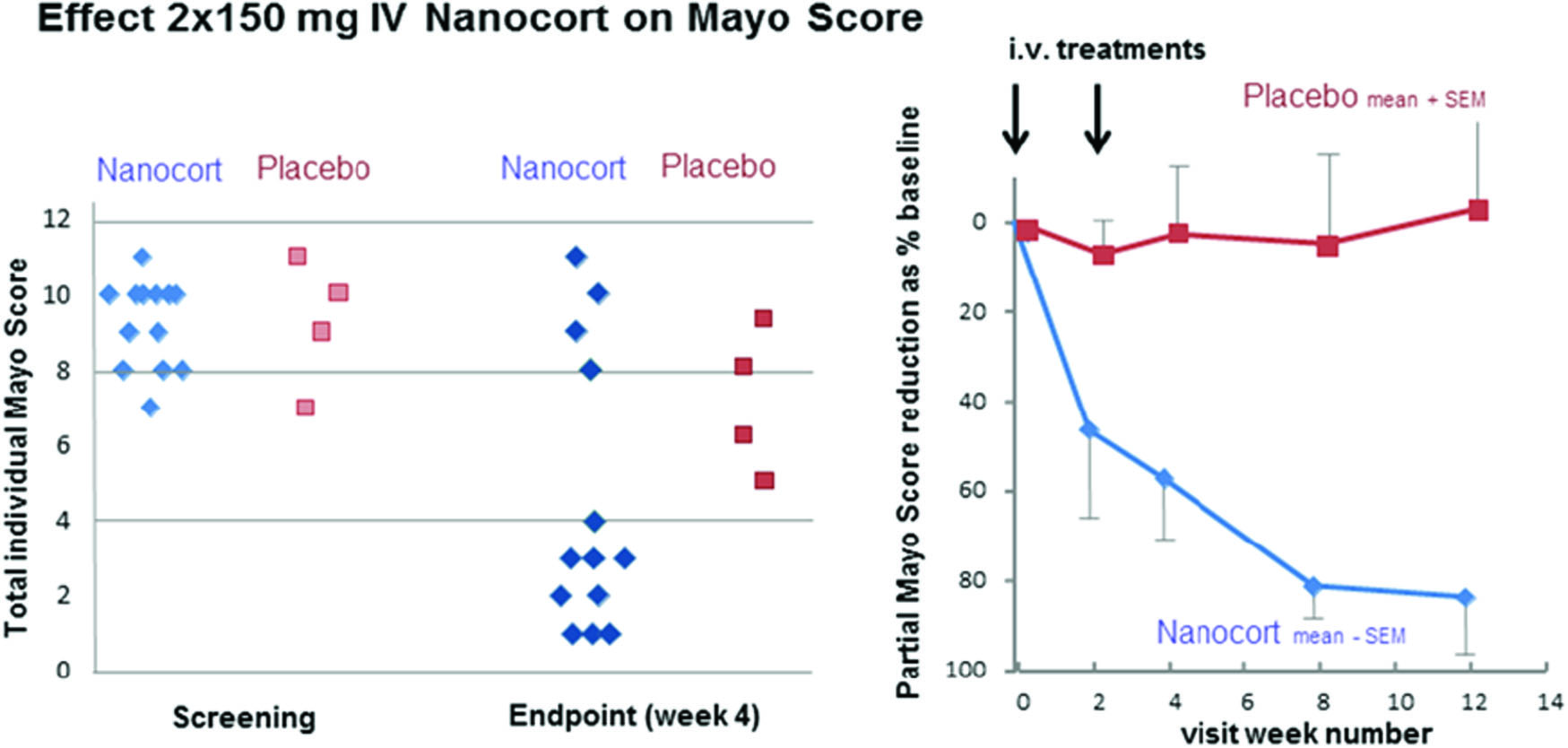
Figure 1. Nanocort effect.
DOP024
Electrical vagus nerve stimulation as an innovative treatment in inflammatory bowel diseases
V. Sinniger1, 2, 3, S. Pellissier3, 4, D. Hoffmann5, C. Trocmé6, L. Vercueil3, 7, D. Clarençon3, 8, B. Bonaz*3, 9
1Hospital University Centre, Hepatogastroenterology, Grenoble Cx9, France, 2Hospital University Centre, Gastroenterology, Grenoble Cx9, France, 3Inserm U836, Grenoble, France, 4University of savoie Mont Blanc, Psychology department, chambéry, France, 5Hospital University Centre, Neurosurgery, Grenoble, France, 6Hospital University Centre, Biology, Grenoble, France, 7Hospital University Centre, Neurology, Grenoble Cx9, France, 8Research Institute of the French Army, Brétigny-sur-Orge, France, 9Hospital University Centre, Gastroenterology, Grenoble, France
DOP025
Clinical response to anti-MMP9 antibody (GS-5745) is accompanied by histologic improvement in ulcerative colitis
W. Sandborn1, 2, B. Bhandari3, R. Fogel4, J. Onken5, E. Yen6, E. Huntzicker6, Y. Xin6, D. French6, J. Silverman6, B. Kanwar6, M. Subramanian6, J. McHutchison6, S. Lee7, L. Shackelton8, L. Stitt8, R. Pai9, B. Levesque*2, G. D’Haens10, 11, B. Feagan8, 12
1University of California, San Diego, California, United States, 2Robarts Clinical Trials, San Diego, California, United States, 3Delta Research Partners, Monroe, Louisiana, United States, 4Clinical Research Institute of Michigan, LLC, Chesterfield, Michigan, United States, 5Duke University Medical Centre, Durham, North Carolina, United States, 6Gilead Sciences, Inc, Foster City, California, United States, 7University of Washington, Seattle, Washington, United States, 8Robarts Clinical Trials, London, Ontario, Canada, 9Mayo Clinic Arizona, Scottsdale, Arizona, United States, 10Academic Medical Centre, Amsterdam, Canada, 11Robarts Clinical Trials, Amsterdam, Netherlands, 12University of Western Ontario, London, Ontario, Canada
DOP026
The Toll-like-receptor 9 agonist DIMS0150 demonstrates therapeutic efficacy for the patient-reported outcome measures PRO-2 and ClinPRO in moderate-to-severe active ulcerative colitis
R. Atreya*1, S. Bloom2, F. Scaldaferi3, V. Gerardi4, C. Admyre5, A. Karlsson5, T. Knittel5, J. Kowalski5, M. Lukas6, R. Löfberg7, R. Petryka8, R. Schnabel9, U. Seidler10, S. Nancey11, M. Neurath1, C. Hawkey12
1University of Erlangen-Nuernberg, Department of Medicine 1, Erlangen, Germany, 2University College London Hospital, Department of Gastroenterology, London, United Kingdom, 3Catholic University of Rome, Internal Medicine Department / Gastroenterology Division, Rome, Italy, 4Catholic University of Rome, Rome, Italy, 5Index Pharmaceuticals, Stockholm, Sweden, 6IBD Clinical and Research Centre, Clinical Centre Isacre Lighthouse, Prague, Czech Republic, 7Karolinska Institute and Sophiahemmet, Stockholm, Sweden, 88NZOZ Vivamed, Warsaw, Poland, 9Pannonia Maganorvosi Centrum, Budapest, Hungary, 10MHH, Department of Gastroenterology, Hepatology, and Endocrinology, Hannover, Germany, 11Lyon-Sud Hospital, Department of Gastroenterology, Lyon, France, 12Nottingham University Hospitals, Department of Gastroenterology, Nottingham, United Kingdom
DOP027
Large-scale drug screen reveals benzimidazole anti-helminthics as potential anti-TNF co-therapy
M. Wildenberg*1, A. Levin2, A. Ceroni3, Z. Guo2, F. Bloemendaal2, D. Ebner3, G. van den Brink1
1Academic Medical Centre, Gastroenterology and Hepatology, Amsterdam, Netherlands, 2Academic Medical Centre, Tytgat Institute, Amsterdam, Netherlands, 3Oxford University, Target Discovery Institute, Oxford, United Kingdom
DOP028
Efficacy and safety of biosimilar infliximab after one year: results from a prospective nationwide cohort
K. Gecse*1, Z. Vegh1, Z. Kurti1, M. Rutka2, K. Farkas2, J. Banai3, L. Bene4, B. Gasztonyi5, P. A. Golovics1, T. Kristof6, L. Lakatos7, P. Miheller8, F. Nagy9, K. Palatka10, M. Papp11, L. Lakner12, A. Patai13, A. Salamon14, T. Szamosi3, Z. Szepes9, B. Szalay15, G. T. Toth16, A. Vincze17, T. Molnar2, P. Lakatos1
1Semmelweis University, First Department of Medicine, Budapest, Hungary, 2University of Szeged, First Department of Medicine, Szeged, Hungary, 3Military Hospital, State Health Centre, Department of Gastroenterology, Budapest, Hungary, 4Peterfy Hospital, First Department of Medicine, Budapest, Hungary, 5Zala County Hospital, Second Department of Medicine, Zalaegerszeg, Hungary, 6B-A-Z County and University Teaching Hospital, Second Department of Medicine, Miskolc, Hungary, 7Csolnoky F. Province Hospital, Department of Medicine, Veszprem, Hungary, 8Semmelweis University, Second Department of Medicine, Budapest, Hungary, 9University of Szeged, First Department of Internal Medicine, Szeged, Hungary, 10University of Debrecen, Institute of Medicine, Department of Gastroenterology, Debrecen, Hungary, 11University of Debrecen, Institute of Internal Medicine, Department of Gastroenterology, Debrecen, Hungary, 12Markusovszky Hospital, Department of Medicine and Gastroenterology, Szombathely, Hungary, 13Markusovszky Hospital, First Department of Medicine and Gastroenterology, Szombathely, Iceland, 14Tolna County Teaching Hospital, First Department of Gastroenterology, Szekszard, Hungary, 15Semmelweis University, Department of Laboratory Medicine, Budapest, Hungary, 16Janos Hospital, Department of Gastroenterology, Budapest, Hungary, 17University of Pécs, First Department of Medicine, Pécs, Hungary
DOP029
Outcomes of a managed switching programme changing IBD patients established on originator infliximab to biosimilar infliximab
M. Bettey1, L. Downey1, C. Underhill1, J. Callaghan1, M. Rush2, I. Ahmed1, F. Cummings*1
1University Hospital Southampton NHS Trust, Department of Gastroenterology, Southampton, United Kingdom, 2University of Southampton, Department of Gastroenterology, Southampton, United Kingdom
DOP030
Elective switching from Remicade® to biosimilar CT-P13 in inflammatory bowel disease patients: a prospective observational cohort study
L. Smits*, L. Derikx, J. Drenth, D. de Jong, A. van Esch, F. Hoentjen
Radboud University Medical Centre, Inflammatory Bowel Disease Centre, Department of Gastroenterology and Hepatology, Nijmegen, Netherlands
DOP031
Efficacy of infliximab biosimilar CT-P13 therapy on mucosal healing in ulcerative colitis: data from 2 Central European countries
K. Farkas1, M. Rutka1, P.A. Golovics2, Z. Végh2, B.D. Lovász2, T. Nyári3, K.B. Gecse2, M. Kolar4, 5, M. Bortlik4, 6, D. Duricova4, 7, N. Machkova4, V. Hruba4, M. Lukas4, K. Mitrova4, 8, K. Malickova9, A. Bálint1, F. Nagy1, R. Bor*1, A. Milassin1, Z. Szepes1, K. Palatka10, P.L. Lakatos2, M. Lukas4, 9, T. Molnár1
1University of Szeged, First Department of Medicine, Szeged, Hungary, 2Semmelweis University, First Department of Medicine, Budapest, Hungary, 3University of Szeged, Department of Medical Physics and Informatics, Szeged, Hungary, 4IBD Clinical and Research Centre, Iscare a.s, Prague, Czech Republic, 5Charles University, First Medical Faculty, Prague, Czech Republic, 6Military Hospital, Charles University, Department of Internal Medicine, Prague, Czech Republic, 7 First Medical Faculty, Charles University, Institute of Pharmacology, Prague, Czech Republic, 8Faculty Hospital Motol, 2nd Medical Faculty, Charles University, Department of Paediatrics, Prague, Czech Republic, 9 First Medical Faculty and General Teaching Hospital, Charles University, Institute of Medical Biochemistry and Laboratory Diagnostics, Prague, Czech Republic, 10University of Debrecen, Second Department of Medicine, Debrecen, Hungary
DOP032
Switching of patients with inflammatory bowel disease from original infliximab (Remicade®) to biosimilar infliximab (Remsima™) is effective and safe
M. Kolar*1, D. Duricová2, M. Brotlik1, V. Hruba1, N. Machkova1, K. Mitrova1, K. Malickova3, M. Lukas1, M. Lukas1
1Charles University, IBD Clinical and Research Centre, ISCARE, Prague, Czech Republic, 2Charles University, IBD Clinical and Research Centre, Iscare, Prague, Czech Republic, 3 First Medical Faculty and General Teaching Hospital, Charles University, Institute of Medical Biochemistry and Laboratory Diagnostics, Prague, Czech Republic
DOP033
Immunogenicity profile and predictors of TLs and ADA development of biosimilar infliximab during the first 6 months of the therapy: results from a prospective nationwide cohort
B. Lovasz*1, Z. Kurti1, M. Rutka2, Z. Vegh1, K. Gecse1, K. Farkas2, J. Banai3, L. Bene4, B. Gasztonyi5, T. Kristof6, L. Lakatos7, P. Miheller8, K. Palatka9, A. Patai10, A. Salamon11, T. Szamosi3, Z. Szepes12, E. Biro13, G.T. Toth14, A. Vincze15, T. Molnar2, P. Lakatos1
1Semmelweis University, First Department of Medicine, Budapest, Hungary, 2University of Szeged, First Department of Medicine, Szeged, Hungary, 3Military Hospital, State Health Centre, Department of Gastroenterology, Budapest, Hungary, 4Peterfy Hospital, First Department of Medicine, Budapest, Hungary, 5Zala County Hospital, Second Department of Medicine, Zalaegerszeg, Hungary, 6B-A-Z County and University Teaching Hospital, Second Department of Medicine, Miskolc, Hungary, 7Csolnoky F. Province Hospital, Department of Medicine, Veszprem, Hungary, 8Semmelweis University, Second Department of Medicine, Budapest, Hungary, 9University of Debrecen, Institute of Medicine, Department of Gastroenterology, Debrecen, Hungary, 10Markusovszky Hospital, First Department of Medicine and Gastroenterology, Szombathely, Iceland, 11Tolna County Teaching Hospital, First Department of Gastroenterology, Szekszard, Hungary, 12University of Szeged, First Department of Internal Medicine, Szeged, Hungary, 13Semmelweis University, Department of Laboratory Medicine, Budapest, Hungary, 14Janos Hospital, Department of Gastroenterology, Budapest, Hungary, 15University of Pécs, First Department of Medicine, Pécs, Hungary
DOP034
Comparative effectiveness of infliximab and adalimumab in Crohn’s disease: results from a real-life population-based cohort
S. Jeuring*1, 2, D. Wintjens1, T. Van den Heuvel1, 2, M. Zeegers3, 4, W. Hameeteman1, M. Romberg-Camps5, L. Oostenbrug6, A. Masclee1, 2, D. Jonkers1, 2, M. Pierik1, 2
1Maastricht University Medical Centre, Internal Medicine - Division of Gastroenterology and Hepatology, Maastricht, Netherlands, 2Maastricht University Medical Centre, NUTRIM - School for Nutrition and Translational Research in Metabolism, Maastricht, Netherlands, 3Maastricht University Medical Centre, Complex Genetics - School for Nutrition and Translational Research in Metabolism (NUTRIM), Maastricht, Netherlands, 4Maastricht University Medical Centre, CAPHRI - School for Public Health and Primary Care, Maastricht, Netherlands, 5Zuyderland Medical Centre, Internal Medicine and Gastroenterology-Hepatology, Sittard-Geleen, Netherlands, 6Zuyderland Medical Centre, Internal Medicine and Gastroenterology-Hepatology, Heerlen, Netherlands
DOP035
Long-term outcome of inflammatory bowel disease patients with deep remission after discontinuation of TNFα –blocking agents
P. Molander*1, 2, M. Färkkilä2, 3, H. Kemppainen4, T. Blomster5, A. Jussila6, H. Mustonen7, T. Sipponen2, 3
1Helsinki University Central Hospital, Peijas Hospital, Gastroenterology, Vantaa, Finland, 2University of Helsinki, Helsinki, Finland, 3Helsinki University Central Hospital, Abdominal Centre, Clinic of Gastroenterology, Helsinki, Finland, 4Turku University Central Hospital, Department of Gastroenterology, Turku, Finland, 5Oulu University Hospital, Department of Gastroenterology, Oulu, Finland, 6Tampere University Hospital, Department of Gastroenterology and Alimentary Tract Surgery, Tampere, Finland, 7Helsinki University Central Hospital, Department of Surgery, Helsinki, Finland
DOP036
Infliximab discontinuation is associated with a higher risk for relapse in patients with ulcerative colitis in remission: a multinational collaborative retrospective study
G. Fiorino*1, P. Ellul2, M. Muscat2, P. Karatzas3, M. Silva4, A. Peixoto4, C. Felice5, F. Bossa6, P.L. Lakatos7, S. Sebastian8, B. Ungar9, F. Furfaro1, K. Karmiris10, K.H. Katsanos11, P. Navarro Cortes12, M.M. Boscà Watts12, D.K. Christodoulou11, G. Maconi13, U. Kopylov9, A. Armuzzi5, F. Magro4, G. Mantzaris3, S. Ben-Horin9, S. Danese1, 14
1Humanitas Research Hospital, IBD Centre, Gastroenterology, Rozzano, Milan, Italy, 2Mater Dei Hospital, Department of Gastroenterology, Msida, Malta, 3Evangelismos Hospital, Department of Gastroenterology, Athens, Greece, 4Centro Hospitalar São João, Oporto, Portugal, Gastrenterology, Oporto, Portugal, 5Complesso Integrato Columbus, Gemelli Hospital Catholic University Foundation, IBD Unit, Rome, Italy, 6Casa Sollievo della Sofferenza Hospital, Division of Gastroenterology, San Giovanni Rotondo, Italy, 7Semmelweis University, 1st Department of Medicine, Budapest, Hungary, 8Hull & East Yorkshire NHS Trust, Hull & York Medical School, Hull, United Kingdom, 9Sheba Medical Centre, Gastroenterology, Tel Hashomer, Tel-Aviv, Israel, 10Venizelion General Hospital, Department of Gastroenterology, Heraklion, Greece, 11University Hospital of Ioannina - Greece, Department of Gastroenterology, Ioannina, Greece, 12University Clinic Hospital of Valencia, Department of Gastroenterology, Valencia, Spain, 13Luigi Sacco University Hospital, Department of Gastroenterology, Milan, Italy, 14Humanitas University, Department of Biomedical Sciences, Rozzano, Italy
DOP037
Excess steroid use in IBD: too much, how much, and why? Results from a UK nationwide audit
T. Raine1, A. Bassi2, E. Fogden3, B. Hayee4, J. K. Limdi5, H. Ludlow6, S. McLaughlin7, G. C. Parkes8, P. Patel9, M. Smith10, C. Selinger*11
1Addenbrooke’s Hospital, Cambridge University Hospitals NHS Foundation Trust, Department of Gastroenterology Research, Cambridge, United Kingdom, 2Whiston Hospital, Department of Gastroenterology, Prescot, United Kingdom, 3Sandwell District General Hospital, Department of Gastroenterology, West Bromwich, United Kingdom, 4King’s College Hospital, Department of Gastroenterology, London, United Kingdom, 5Pennine Acute Hospitals NHS Trust, Department of Gastroenterology, Manchester, United Kingdom, 6University Hospital Llandough, Department of Gastroenterology, Penarth, Cardiff, United Kingdom, 7Royal Bournemouth Hospital, Department of Gastroenterology, Bournemouth, United Kingdom, 8Barts and The London, Queen Mary’s School of Medicine and Dentistry, Department of Gastroenterology, London, United Kingdom, 9Epsom General Hospital, Department of Gastroenterology, Epsom, United Kingdom, 10Brighton and Sussex University Hospitals Trust, Digestive Diseases, Hove, East Sussex, United Kingdom, 11St James University Hospital, Gastroenterology, Leeds, United Kingdom
DOP038
Analytical and clinical validation of a rapid point-of-care assay for infliximab quantification in patients with ulcerative colitis
T. Van Stappen*1, L. Bollen1, N. Vande Casteele1, K. Papamichail1, 2, N. Geukens1, C. Barthel3, Y. Kölmel3, N. Zali3, S. Rameil3, G. Van Assche2, M. Ferrante2, S. Vermeire2, A. Gils1
1KU Leuven, Department of Pharmaceutical and Pharmacological Sciences, Leuven, Belgium, 2UZ Leuven, Department of Clinical and Experimental Medicine, Leuven, Belgium, 3R-Biopharm AG, Darmstadt, Germany